In chemistry, the terms “sigma” and “pi” bonds often surface in discussions concerning molecular structures and chemical interactions. These two types of chemical bonds play pivotal roles in shaping the properties and behaviors of molecules. This comprehensive article delves deep into the difference between sigma and pi bonds, shedding light on their definitions, characteristics, formation, and significance. Whether you’re a chemistry enthusiast seeking a detailed understanding or a student aiming to ace your chemistry course, this article is designed to be engaging, easy to comprehend, and packed with valuable insights.
The Basics of Chemical Bonding
Before we dive into sigma and pi bonds, let’s establish a foundational understanding of chemical bonding. At its core, chemistry is the science of matter, and matter is composed of atoms. These atoms, whether in isolation or combination, seek stability by achieving a full complement of electrons in their outermost energy levels. This quest for stability drives the formation of chemical bonds.
Covalent Bonding
One of the most prevalent types of chemical bonding is covalent bonding. In covalent bonds, atoms share electrons to satisfy their electron configuration, resulting in the formation of molecules. Covalent compounds, also known as molecules, are integral to the chemistry of life, industry, and everyday materials.
The Birth of Sigma and Pi Bonds
When atoms come together to form covalent compounds, they don’t just share electrons randomly; there’s a method to the madness. The concept of sigma and pi bonds emerges from the way electrons are shared between atoms in a covalent bond. These bonds are crucial in understanding the structures and reactivity of various chemical compounds.
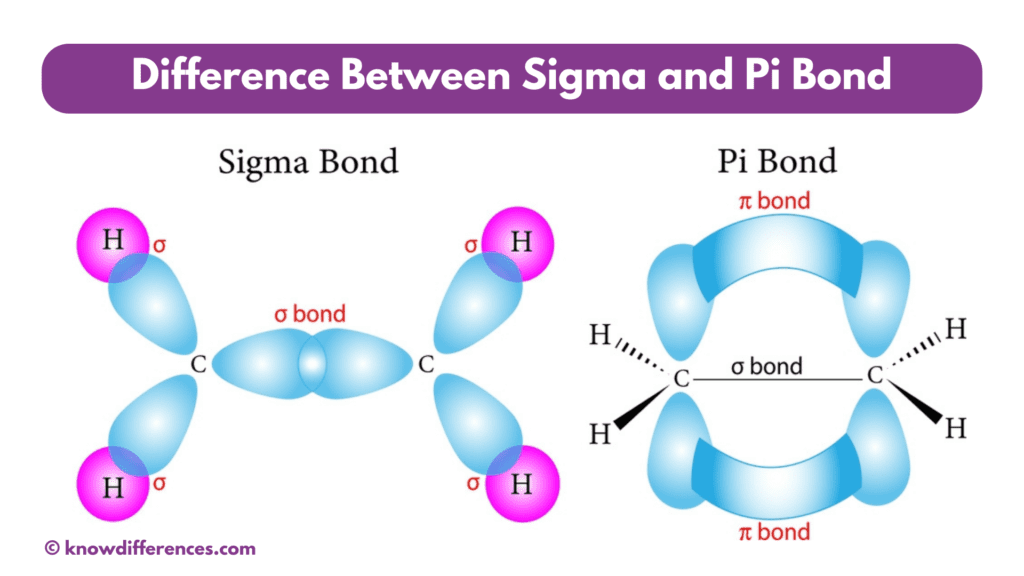
Sigma vs Pi Bond: Quick Overview
The main difference between sigma and pi bonds lies in their bonding orientation: sigma bonds are formed by the overlap of atomic orbitals head-on, while pi bonds result from the lateral overlap of p orbitals.
Here’s a table highlighting the key differences between sigma (σ) and pi (π) bonds:
| Characteristic | Sigma (σ) Bond | Pi (π) Bond |
|---|---|---|
| Bond Type | Single bonds have one σ bond | Double and triple bonds have one σ bond and one or two π bonds, respectively |
| Overlap of Atomic Orbitals | Head-on overlap of atomic orbitals | Side-to-side overlap of atomic orbitals |
| Strength | σ bonds are generally stronger | π bonds are weaker than σ bonds |
| Bonding Electrons | Shared directly along the bond axis | Shared above and below the bond axis |
| Shape | Cylindrical or linear shape | Doughnut or figure-eight shape |
| Rotation | Allows free rotation around the bond axis | Restricts rotation due to the presence of the π bond |
| Hybridization | σ bonds often involve sp, sp2, or sp3 hybridization | π bonds usually involve unhybridized p orbitals |
| Multiple Bonds | Present in all covalent bonds, including single bonds | Present in double and triple bonds |
| Bond Strength | σ bonds contribute significantly to bond strength | π bonds contribute less to bond strength |
This table summarizes the primary differences between sigma and pi bonds, which are two types of covalent bonds formed in molecular compounds. Sigma bonds result from head-on overlap, while pi bonds result from side-to-side overlap of atomic orbitals. Understanding these differences is crucial in the study of chemical bonding and molecular structure.
Characteristics of Sigma Bonds
Sigma (σ) bonds are the primary or “single” bonds that connect atoms in a molecule. These bonds are the strongest type of covalent bonds and are characterized by several key features:
- End-to-End Overlap: Sigma bonds are formed by the direct head-on overlap of atomic orbitals. This means that the electron density is concentrated on the bond axis, resulting in a strong bond.
- Rotational Freedom: Molecules connected by sigma bonds exhibit free rotation around the bond axis. This is because the electron density is evenly distributed along the axis, allowing atoms to spin without breaking the bond.
- Single Bonds: Sigma bonds are typically associated with single bonds in molecular structures. Single bonds are the simplest form of covalent bonds, involving the sharing of one pair of electrons between two atoms.
- Spherical Symmetry: The electron density in a sigma bond is distributed symmetrically, giving it a spherical shape around the bond axis.
Formation of Sigma Bonds
The process of forming sigma bonds involves the overlap of atomic orbitals. This overlap is most commonly observed in s-s and s-p hybridized orbitals, as well as p-p orbitals. Let’s delve into the details of sigma bond formation:
- s-s Overlap: When two s-orbitals overlap, they create a sigma bond. For example, in a diatomic hydrogen (H2) molecule, two hydrogen atoms each with a 1s orbital overlap to form a sigma bond.
- s-p Overlap: When an s-orbital overlaps with a p-orbital, it results in a sigma bond. This type of overlap is commonly seen in molecules like ammonia (NH3), where the nitrogen atom’s 2s orbital overlaps with one of its 2p orbitals.
- p-p Overlap: When two p-orbitals overlap head-on, they form a sigma bond. A classic example is the sigma bond between the carbon atoms in ethene (C2H4), a compound in which carbon-carbon double bonds play a pivotal role.
Characteristics of Pi Bonds
While sigma bonds form the foundation of covalent compounds, pi (π) bonds are an integral part of double and triple bonds. Pi bonds exhibit distinct characteristics that set them apart from sigma bonds:
- Side-to-Side Overlap: Unlike sigma bonds, pi bonds are formed by the lateral or side-to-side overlap of two parallel p-orbitals. This results in the electron density being concentrated above and below the bond axis.
- Double and Triple Bonds: Pi bonds are commonly associated with double (e.g., C=C) and triple (e.g., C≡C) bonds. In these cases, the sharing of electrons involves both sigma and pi bonds.
- Limited Rotation: Molecules connected by pi bonds have restricted rotation around the bond axis. The electron density is concentrated in a region above and below the bond, preventing free rotation.
- P-Distribution: In pi bonds, the electron density is distributed in a dumbbell-shaped region above and below the bond axis, creating a cloud-like appearance.
Formation of Pi Bonds
Pi bond formation is distinct from sigma bond formation, as it involves the lateral overlap of parallel p-orbitals. Let’s explore how pi bonds are created:
- p-p Overlap: Pi bonds primarily result from the head-on overlap of two parallel p-orbitals. In compounds like ethene (C2H4), pi bonds form between carbon atoms through the lateral overlap of their 2p orbitals.
- Double Bonds: In molecules with double bonds, such as oxygen (O2), pi bonds are formed in addition to sigma bonds. Oxygen molecules consist of a sigma bond and two pi bonds, contributing to the stability of the molecule.
- Triple Bonds: In compounds with triple bonds, such as nitrogen (N2), there is one sigma bond and two pi bonds between the nitrogen atoms, creating a highly stable molecular structure.
Key Differences Between Sigma and Pi Bonds
The main differences between sigma and pi bonds are as follows:
- Overlap: Sigma bonds involve head-on overlap of atomic orbitals, while pi bonds involve side-to-side overlap of parallel p-orbitals.
- Strength: Sigma bonds are stronger due to the direct orbital overlap, while pi bonds are relatively weaker due to lateral overlap and greater electron repulsion.
- Rotation: Sigma bonds allow free rotation within the bond, while pi bonds restrict rotation due to electron density above and below the bond axis.
- Shape: Sigma bonds have spherical symmetry around the bond axis, whereas pi bonds have a dumbbell-shaped distribution above and below the bond axis.
- Bond Multiplicity: Sigma bonds are typically associated with single bonds, while pi bonds are present in double and triple bonds, contributing to the rigidity of these bonds.
When Sigma and Pi Bonds Coexist
In many covalent compounds, both sigma and pi bonds coexist to create complex molecular structures. Understanding their coexistence is crucial for appreciating the stability and reactivity of such compounds. Here are some common scenarios:
- Double Bonds: Double bonds, such as those found in alkenes (e.g., ethene), consist of one sigma bond and one pi bond between the bonded atoms. The pi bond contributes to the rigidity of the double bond, preventing free rotation.
- Triple Bonds: Triple bonds, like those in alkynes (e.g., ethyne), contain one sigma bond and two pi bonds. The presence of two pi bonds makes triple bonds even stronger and less prone to rotation.
- Aromatic Compounds: Aromatic compounds, exemplified by benzene, feature a unique ring structure with alternating single and double bonds. These delocalized pi bonds contribute to the stability and resonance of the molecule.
- Resonance Structures: In some molecules, resonance structures involve the redistribution of electrons, leading to the formation of both sigma and pi bonds in different resonance forms. An example is the carbonate ion (CO3²⁻).
Let’s go through the practice problems and count the sigma and pi bonds in each molecule, along with explanations:
Problem 1: Ethene (C2H4)
- Ethene (C2H4) consists of a carbon-carbon double bond. In this molecule, each carbon forms one sigma bond with hydrogen (C-H) and one sigma bond with the other carbon (C-C), making a total of four sigma bonds. Additionally, there is one pi bond between the two carbon atoms, which results from the side-to-side overlap of their p-orbitals.
- Sigma Bonds: 4
- Pi Bonds: 1
Problem 2: Nitrogen Gas (N2)
- Nitrogen gas (N2) is composed of a nitrogen-nitrogen triple bond. In this molecule, each nitrogen atom forms one sigma bond with the other nitrogen atom (N≡N), and there are two additional pi bonds between the two nitrogen atoms. These pi bonds result from the side-to-side overlap of p-orbitals.
- Sigma Bonds: 1 (single sigma bond in N≡N)
- Pi Bonds: 2
Problem 3: Benzene (C6H6)
- Benzene (C6H6) is a hexagonal aromatic ring with alternating single and double bonds. The alternating double bonds give rise to three pi bonds, while each carbon-hydrogen bond forms a sigma bond. In benzene, there are six carbon-carbon sigma bonds and three carbon-carbon pi bonds.
- Sigma Bonds: 6
- Pi Bonds: 3
Problem 4: Acetylene (C2H2)
- Acetylene (C2H2) consists of a carbon-carbon triple bond. In this molecule, each carbon atom forms one sigma bond with hydrogen (C-H) and one sigma bond with the other carbon atom (C≡C). There are two additional pi bonds between the two carbon atoms, resulting from the side-to-side overlap of p-orbitals.
- Sigma Bonds: 2 (two C-H sigma bonds)
- Pi Bonds: 2
Problem 5: Ozone (O3)
- Ozone (O3) contains a bent, V-shaped structure. Each oxygen atom in ozone forms a sigma bond with the central oxygen atom, and there are two pi bonds between the central oxygen atom and the other two oxygen atoms. These pi bonds result from the side-to-side overlap of p-orbitals.
- Sigma Bonds: 2 (two O-O sigma bonds)
- Pi Bonds: 2
Problem 6: Formaldehyde (CH2O)
- Formaldehyde (CH2O) is composed of a carbon-oxygen double bond. In this molecule, the carbon atom forms a sigma bond with each of the two hydrogen atoms (C-H), and it also forms a sigma bond with the oxygen atom (C=O). Additionally, there is one pi bond between the carbon and oxygen atoms, resulting from the side-to-side overlap of p-orbitals.
- Sigma Bonds: 3 (two C-H sigma bonds and one C=O sigma bond)
- Pi Bonds: 1
These practice problems explanations should help you understand how to count sigma and pi bonds in various chemical compounds. Remember that sigma bonds involve head-on overlap of atomic orbitals, while pi bonds result from the lateral overlap of parallel p-orbitals.
Applications and Significance
Biological Implications
The understanding of sigma and pi bonds is not limited to the laboratory or industrial settings; it also extends to the realm of biology. These bonds play critical roles in the structure and function of biomolecules. Let’s explore their biological significance:
- Proteins and Enzymes: Sigma and pi bonds are essential for maintaining the three-dimensional structures of proteins and enzymes. Hydrogen bonds, a type of sigma bond, are key in stabilizing protein structures.
- Nucleic Acids: In DNA and RNA, pi bonds are involved in the stacking of aromatic nucleobases. These interactions are crucial for the structural stability and base-pairing in the genetic code.
- Enzymatic Reactions: Enzymes catalyze chemical reactions in living organisms, and their mechanisms often rely on the interaction of sigma and pi bonds with substrates and coenzymes.
- Cell Membranes: Lipids, which are vital components of cell membranes, contain both sigma and pi bonds. The properties of these bonds influence the fluidity and permeability of cell membranes.
Industrial and Technological Relevance
Beyond biology, the distinction between sigma and pi bonds holds immense importance in various industrial and technological applications. Here’s how these bonds impact our daily lives:
- Chemical Synthesis: Chemists use the knowledge of sigma and pi bonds to design and synthesize new compounds, drugs, and materials with specific properties.
- Materials Science: The properties of materials, such as polymers and plastics, are influenced by the presence of sigma and pi bonds. These bonds determine characteristics like strength, flexibility, and electrical conductivity.
- Catalysis: Many industrial processes rely on catalysis, where catalysts interact with reactants through sigma and pi bonds. Examples include the catalytic converters in automobiles and the production of chemicals in refineries.
- Semiconductors: The electronic properties of semiconductors are closely tied to the behavior of pi bonds, making them essential in modern technology, including electronics and computing.
- Pharmaceuticals: Drug development often involves the manipulation of sigma and pi bonds to create compounds with specific biological activities and minimal side effects.
Challenges and Limitations
Bond Strength and Reactivity
While sigma and pi bonds are integral to the stability of covalent compounds, they also pose certain challenges and limitations. Understanding these aspects is crucial for addressing specific issues in chemical reactions and materials science:
- Bond Strength: Sigma bonds are generally stronger than pi bonds due to the direct overlap of atomic orbitals. This strength can make it difficult to break sigma bonds in chemical reactions, requiring significant energy input.
- Reactivity: Pi bonds are relatively weaker, and their electron density is distributed above and below the bond axis. This distribution can lead to higher reactivity, making pi bonds susceptible to attack by electrophiles and nucleophiles.
- Selectivity: The presence of both sigma and pi bonds in a molecule can impact the selectivity of chemical reactions. For example, in synthesis, it may be challenging to target specific sigma or pi bonds in a complex molecule.
- Steric Hindrance: The presence of multiple sigma and pi bonds in close proximity can lead to steric hindrance, where the bulkiness of substituents hinders reaction pathways. Chemists often need to consider steric effects in complex reactions.
The Role of Hybridization
Hybridization of atomic orbitals is a concept closely related to the formation of sigma and pi bonds. Hybridization occurs when atomic orbitals mix to create new hybrid orbitals, which influences the geometry and bonding in molecules. Some important considerations regarding hybridization include:
- Hybrid Orbital Types: Different types of hybrid orbitals, such as sp, sp2, and sp3, contribute to the formation of sigma and pi bonds. The choice of hybridization depends on the geometry of the molecule.
- Geometry and Bonding: The hybridization of orbitals determines the shape of a molecule and the angles between sigma bonds. For example, in methane (CH4), sp3 hybridization leads to a tetrahedral shape.
- Pi Bonding and Hybridization: Pi bonds form from unhybridized p-orbitals. In compounds with pi bonds, it’s common to have sigma bonds formed from hybridized orbitals and pi bonds formed from unhybridized p-orbitals.
- Influence on Molecular Properties: Hybridization affects molecular properties such as bond angles, bond lengths, and polarity, all of which have implications for a molecule’s reactivity and behavior.
FAQs: Frequently Asked Questions
How do you count sigma and pi bonds?
To count sigma and pi bonds in a molecule, follow these steps:
First, draw the Lewis structure of the molecule to visualize the bonds.
Examine each bond in the molecule.
If the bond involves the head-on overlap of atomic orbitals, it’s a sigma bond (σ).
If the bond involves the side-to-side overlap of parallel p-orbitals, it’s a pi bond (π).
Count the number of sigma and pi bonds in the molecule based on your analysis.
How do you identify sigma and pi bonds?
Sigma bonds (σ) are identified by the head-on overlap of atomic orbitals between two atoms. In a sigma bond, the electron density is concentrated along the bond axis, and the bond allows free rotation.
Pi bonds (π) are identified by the side-to-side overlap of parallel p-orbitals between two atoms. In a pi bond, the electron density is distributed above and below the bond axis, restricting rotation.
Which is stronger, sigma or pi bond, and why?
Sigma bonds are generally stronger than pi bonds. The strength of a covalent bond is determined by the extent of orbital overlap. Sigma bonds involve direct head-on overlap of atomic orbitals, resulting in a more concentrated bond along the bond axis. In contrast, pi bonds involve side-to-side overlap, leading to weaker bonding and increased electron repulsion. As a result, sigma bonds require more energy to break in chemical reactions, making them stronger.
Why is a sigma bond formed first?
In the formation of a covalent bond, a sigma (σ) bond is typically formed first because it represents the direct head-on overlap of atomic orbitals. This type of overlap allows the atoms to get closer to each other, resulting in a stronger bond. Once a sigma bond is established, any additional bonds between the same atoms (e.g., in double or triple bonds) involve pi (π) bonds, which form from the side-to-side overlap of parallel p-orbitals.
Conclusion
The difference between sigma and pi bonds is a fundamental concept in chemistry, providing valuable insights into the behavior of covalent compounds. Sigma bonds, characterized by direct head-on overlap of atomic orbitals, serve as the foundation of single bonds and enable free rotation in molecules. Pi bonds, on the other hand, result from side-to-side overlap of parallel p-orbitals and are essential in double and triple bonds, offering rigidity and restricted rotation.
Understanding the differences between sigma and pi bonds is not only crucial in the academic realm but also has far-reaching applications in biology, industry, and technology. These bonds influence the stability and function of biomolecules, the design of materials with specific properties, catalytic processes, and the development of pharmaceuticals.
While sigma and pi bonds are indispensable, they also present challenges, such as variations in bond strength and reactivity, as well as the influence of hybridization on molecular properties.
References
- McMurry, J., & Fay, R. C. (2004). Chemistry (4th ed.). Pearson.
- Brown, T. L., LeMay, H. E., Bursten, B. E., & Burdge, J. R. (2008). Chemistry: The Central Science (11th ed.). Pearson.
- Silberberg, M. S. (2017). Chemistry: The Molecular Nature of Matter and Change (8th ed.). McGraw-Hill.
- Atkins, P., & de Paula, J. (2014). Atkins’ Physical Chemistry (10th ed.). Oxford University Press.
- Vollhardt, K. P. C., & Schore, N. E. (2018). Organic Chemistry: Structure and Function (8th ed.). W. H. Freeman.
- Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry (2nd ed.). Oxford University Press.
- March, J. (1992). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.). Wiley.
- Smith, J. G., & March, J. (2007). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.). Wiley.