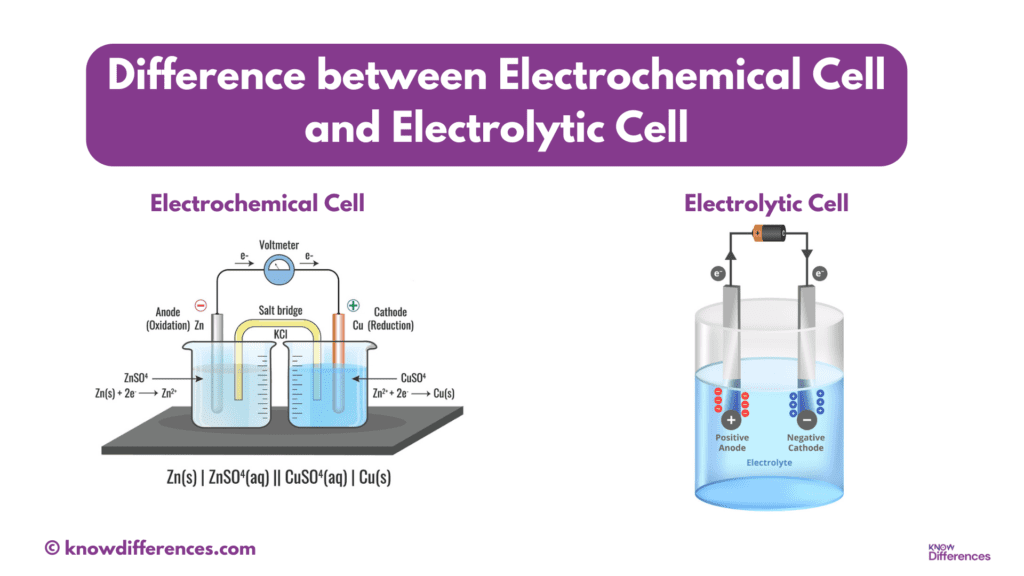
In electrochemistry, two fundamental concepts stand out prominently – the electrochemical cell and the electrolytic cell. These two devices serve distinct purposes, despite sharing similarities in their names and basic operational principles. Understanding the difference between an electrochemical cell and an electrolytic cell is crucial for comprehending their applications in various fields, from chemistry and industry to our daily lives.
Table of Contents
The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: an electrochemical cell converts chemical energy into electrical energy through spontaneous reactions, while an electrolytic cell drives non-spontaneous reactions using electrical energy.
| Feature | Electrochemical Cell | Electrolytic Cell |
|---|---|---|
| Purpose | Generates electrical energy from chemical reactions | Drives non-spontaneous chemical reactions using electrical energy |
| Energy Conversion | Converts chemical energy into electrical energy | Converts electrical energy into chemical energy |
| Spontaneity | Spontaneous reactions occur | Non-spontaneous reactions occur |
| Anode/Cathode | Anode undergoes oxidation, cathode undergoes reduction | Anode undergoes reduction, cathode undergoes oxidation |
| External Power Supply | Not required | Required |
| Electrolyte | Facilitates ion flow between electrodes | Facilitates ion flow between electrodes |
| Application | Batteries, fuel cells | Electroplating, electrolysis |
| Chemical Reactions | Occur spontaneously | Driven by external electrical energy |
| Example | Voltaic cell, Daniell cell | Electrolysis of water, electroplating |
This table summarizes the key differences between electrochemical cells and electrolytic cells, highlighting their purpose, energy conversion mechanisms, spontaneity of reactions, electrode roles, need for external power supply, electrolyte function, applications, and the nature of chemical reactions occurring within each type of cell.
What is an Electrochemical Cell?
An electrochemical cell is a device that converts chemical energy into electrical energy through a spontaneous redox (reduction-oxidation) reaction. It is a fundamental concept in electrochemistry and plays a pivotal role in powering numerous devices, from the batteries in your remote control to the complex electrochemical processes in the human body.
Structure and Components of an Electrochemical Cell
An electrochemical cell consists of several key components:
- Electrodes: These are conductive materials (usually metals or metal compounds) that facilitate electron transfer during the redox reactions. There are two types of electrodes – the anode and the cathode.
- Electrolyte: This is a solution that allows the flow of ions between the anode and cathode, completing the electrical circuit. It may be an aqueous solution, a molten salt, or any medium that contains ions.
- Salt Bridge: A salt bridge is a vital component that maintains electrical neutrality in the cell. It allows the movement of ions between the two half-cells, preventing the buildup of charge and enabling a continuous flow of electrons.
- External Circuit: The electrical energy generated by the redox reaction in the cell is harnessed through an external circuit. This is where the cell’s energy is used to perform work or power electrical devices.


How does an Electrochemical Cell Work?
Electrochemical cells operate on the principles of redox reactions. The anode, where oxidation occurs, releases electrons into the external circuit, while the cathode, where reduction takes place, accepts these electrons. The flow of electrons through the external circuit creates an electric current.
The overall chemical reaction in an electrochemical cell can be represented by a balanced chemical equation. For example, in a common alkaline battery:
Anode: Zn(s) → Zn^2+(aq) + 2e^-
Cathode: 2MnO2(s) + 2H2O(l) + 2e^- → Mn2O3(s) + 2OH^-(aq)
Overall Reaction: Zn(s) + 2MnO2(s) + 2H2O(l) → Zn^2+(aq) + Mn2O3(s) + 2OH^-(aq)
Types of Electrochemical Cells
Electrochemical cells come in various forms, each tailored to specific applications. Some of the most common types include:
- Galvanic (Voltaic) Cells: These are commonly found in batteries, where the chemical reactions release electrical energy. Galvanic cells are the driving force behind portable power sources.
- Electrolytic Cells: Unlike galvanic cells, electrolytic cells require an external voltage source to drive a non-spontaneous chemical reaction. They are often used for processes like electroplating and water electrolysis.
- Fuel Cells: Fuel cells generate electricity through the electrochemical reaction between a fuel and an oxidizer. They are highly efficient and used in applications ranging from powering vehicles to providing backup power.
- Concentration Cells: These cells utilize differences in ion concentrations to produce electrical energy. They are important in chemical analysis and sensors.
- Biological Cells: Living organisms use biological cells to generate and control electrical potentials. Nerve cells, for instance, rely on ion gradients to transmit signals.
Applications of Electrochemical Cells
Electrochemical cells have a wide range of applications:
- Consumer Electronics: Portable electronic devices, such as smartphones and laptops, rely on small electrochemical cells (batteries) to provide power.
- Transportation: Electric vehicles use advanced fuel cells to convert hydrogen into electricity for propulsion.
- Medicine: Implantable medical devices like pacemakers are powered by small batteries.
- Chemical Industry: Electrochemical cells are used in various industrial processes, such as metal plating, where they play a key role in electroplating metals onto surfaces.
- Environmental Protection: Electrochemical cells are used in water treatment to remove contaminants through processes like electrocoagulation.
Now that we have a solid understanding of electrochemical cells, let’s explore their counterpart, electrolytic cells.
What is an Electrolytic Cell?
An electrolytic cell is a device that uses electrical energy from an external source to drive a non-spontaneous redox reaction. Unlike electrochemical cells, which generate electrical energy, electrolytic cells consume electrical energy to cause a chemical change. Electrolysis, the process by which these cells operate, is integral to various industries and scientific experiments.
Structure and Components of an Electrolytic Cell
An electrolytic cell shares some common components with electrochemical cells, but it also has some key differences:
- Electrodes: Similar to electrochemical cells, electrolytic cells have two electrodes – an anode and a cathode. However, the roles of these electrodes are reversed compared to electrochemical cells.
- Electrolyte: The electrolyte in an electrolytic cell is often a non-reactive substance that does not participate in the chemical reaction but serves as a medium for ion transport.
- External Voltage Source: An external power source, such as a battery or power supply, is connected to the electrolytic cell to provide the necessary electrical energy for the non-spontaneous reaction.


How does an Electrolytic Cell Work?
Electrolytic cells operate by applying an external voltage that forces a non-spontaneous chemical reaction to occur. This process is known as electrolysis. The anode, connected to the positive terminal of the external power source, attracts negatively charged ions (anions) from the electrolyte. These anions lose electrons and form neutral atoms or molecules, which are released. At the cathode, connected to the negative terminal of the power source, positively charged ions (cations) gain electrons and form neutral atoms or molecules. The net result is a chemical change driven by the electrical energy supplied.
For instance, in the electrolysis of water:
Anode: 2H2O(l) → O2(g) + 4H^+(aq) + 4e^-
Cathode: 4H^+(aq) + 4e^- → 2H2(g)
Overall Reaction: 2H2O(l) → 2H2(g) + O2(g)
This process separates water into hydrogen and oxygen gas, which has important applications, including in the production of hydrogen for fuel.
Types of Electrolytic Cells
Electrolytic cells can vary in design and purpose. Common types of electrolytic cells include:
- Water Electrolysis Cells: These cells are used to split water into hydrogen and oxygen gases for various industrial and scientific applications, including fuel production and laboratory experiments.
- Electroplating Cells: Electroplating is the process of depositing a layer of one metal onto the surface of another. Electroplating cells are crucial in industries such as jewelry making, automotive manufacturing, and electronics.
- Electrowinning Cells: Electrowinning is the process of extracting metals from ores using electrical energy. It is commonly used in mining and metallurgy.
- Chlor-Alkali Cells: These cells are employed in the production of chlorine gas, sodium hydroxide (caustic soda), and hydrogen gas. They have applications in the chemical industry.
- Electrolytic Capacitors: Electrolytic capacitors are a type of capacitor used in electronics for energy storage and signal filtering.
Applications of Electrolytic Cells
Electrolytic cells have diverse applications across multiple fields:
- Hydrogen Production: Electrolysis of water is a key method for producing hydrogen gas, which is used in fuel cells, the food industry, and as a clean energy carrier.
- Metal Finishing: Electroplating cells are widely used to improve the appearance and durability of various products, from jewelry to car parts.
- Extraction of Metals: Electrowinning cells are essential in the extraction of metals from their ores, a crucial step in the production of metals like copper and aluminum.
- Chemical Manufacturing: Chlor-alkali cells play a significant role in the production of chemicals like chlorine and sodium hydroxide, which are used in various industrial processes.
- Energy Storage: Electrolytic capacitors are used in electronic circuits to store and release electrical energy efficiently.
With a solid understanding of both electrochemical and electrolytic cells, we can now explore the key differences between these two concepts.
Key Differences
Electrical Nature
Electrochemical Cell: Operates with a spontaneous redox reaction that converts chemical energy into electrical energy without the need for an external power source.
Electrolytic Cell: Requires an external power source to drive a non-spontaneous redox reaction, consuming electrical energy to induce a chemical change.
Spontaneity
Electrochemical Cell: Spontaneous redox reactions occur naturally, generating electrical energy as a byproduct.
Electrolytic Cell: Non-spontaneous redox reactions only occur when an external voltage is applied, and electrical energy is consumed in the process.
Purpose
Electrochemical Cell: Designed to provide electrical energy for powering devices, such as batteries and fuel cells.
Electrolytic Cell: Used for various purposes, including electroplating, water electrolysis, metal extraction, and chemical synthesis.
Redox Reactions
Electrochemical Cell: Involves spontaneous redox reactions where the anode undergoes oxidation, and the cathode undergoes reduction.
Electrolytic Cell: Involves non-spontaneous redox reactions where the anode undergoes reduction, and the cathode undergoes oxidation.
External Voltage Source
Electrochemical Cell: Operates without the need for an external voltage source; it generates its own electrical energy.
Electrolytic Cell: Requires an external voltage source to drive the non-spontaneous redox reaction.
Understanding these differences is essential, as it distinguishes the primary functions of electrochemical and electrolytic cells. While they share fundamental principles, their applications and roles in various fields are distinct.
Similarities between Electrochemical and Electrolytic Cells
Despite their differences, electrochemical and electrolytic cells share several common features:
- Redox Reactions: Both types of cells involve redox reactions, where electrons are transferred between the anode and cathode.
- Flow of Electrons: In both cell types, the flow of electrons through an external circuit is essential for the operation of the cell.
- Chemical Transformations: Chemical reactions occur at the electrodes in both types of cells, leading to changes in the composition of substances in the cell.
These similarities underscore the underlying principles of electrochemistry that govern the behavior of both types of cells.
Practical Examples
Battery vs. Electroplating
To illustrate the differences between electrochemical and electrolytic cells, let’s compare the operation of a battery (an electrochemical cell) and a process like electroplating (an electrolytic cell).
Battery (Electrochemical Cell):
- In a typical alkaline battery, the anode consists of zinc (Zn) and the cathode includes manganese dioxide (MnO2) and an electrolyte. The spontaneous redox reaction between these materials generates electrical energy, which powers devices like flashlights.
- The battery operates without the need for an external voltage source, as the redox reaction occurs spontaneously.
Electroplating (Electrolytic Cell):
- In electroplating, a metal object is coated with a layer of another metal, such as silver or gold. This process involves an electrolytic cell.
- The object to be plated serves as the cathode, and a metal salt solution serves as the electrolyte. A power supply is connected to the electrodes.
- The non-spontaneous redox reaction at the anode causes metal ions from the electrolyte to deposit onto the cathode, resulting in a plated layer.
This comparison highlights the distinct purposes and operational principles of electrochemical and electrolytic cells. Batteries generate electrical energy, while electrolytic cells induce non-spontaneous chemical changes.
Fuel Cells vs. Water Splitting
Another pair of examples that exemplify the differences between electrochemical and electrolytic cells are fuel cells and the electrolysis of water.
Fuel Cells (Electrochemical Cell):
- Fuel cells are electrochemical cells that generate electrical energy through the spontaneous redox reaction between a fuel (e.g., hydrogen) and an oxidizer (e.g., oxygen).
- The redox reaction at the anode produces electrons, which flow through an external circuit, powering electrical devices.
Electrolysis of Water (Electrolytic Cell):
- Electrolysis of water is an electrolytic process used to split water into hydrogen and oxygen gases.
- An external voltage source is connected to the electrolytic cell, causing the non-spontaneous redox reaction. At the anode, water molecules are oxidized to release oxygen gas, while at the cathode, water molecules are reduced to form hydrogen gas.
These examples emphasize the distinction between electrochemical cells that produce electrical energy and electrolytic cells that consume electrical energy to drive a chemical change.
Importance in Everyday Life
Understanding the difference between electrochemical and electrolytic cells is not just a matter of theoretical knowledge; it has significant practical implications in our daily lives. These cells play critical roles in various aspects of modern society.
Batteries in Consumer Electronics
Electrochemical cells, particularly batteries, are ubiquitous in consumer electronics. From your smartphone and laptop to remote controls and wristwatches, batteries power a wide range of devices that we rely on daily. The ability of electrochemical cells to provide portable and readily available energy storage has revolutionized the way we live and work.
Electrolysis in Metal Refining
In the industrial sector, electrolytic cells are instrumental in processes such as metal refining and electroplating. For instance, the extraction of metals like aluminum and copper from their ores is achieved through electrowinning, a process that involves the use of large-scale electrolytic cells. Additionally, electroplating plays a crucial role in enhancing the appearance and durability of products, including jewelry, car parts, and consumer electronics.
Medical Applications
Electrochemical cells have made significant contributions to the field of medicine. Implantable medical devices, such as pacemakers, rely on compact batteries to deliver life-saving electrical impulses to the heart. These devices, powered by electrochemical cells, have improved the quality of life and longevity of countless individuals.
Environmental Significance
Electrochemical and electrolytic cells also have implications for environmental protection. Water treatment facilities use electrochemical cells for processes like electrocoagulation, which helps remove contaminants and pollutants from wastewater. Additionally, the development of fuel cells and hydrogen production through water electrolysis holds promise as a clean and sustainable energy source, contributing to efforts to reduce greenhouse gas emissions and combat climate change.
Conclusion
In conclusion, the difference between electrochemical and electrolytic cells lies in their fundamental purpose and operation. Electrochemical cells harness spontaneous redox reactions to generate electrical energy, while electrolytic cells use external voltage sources to drive non-spontaneous chemical changes. These distinctions have far-reaching implications, from the batteries that power our daily gadgets to the electrolytic processes that shape industries and contribute to environmental sustainability.
Understanding these differences not only deepens our appreciation for the world of electrochemistry but also equips us with the knowledge to make informed decisions and advancements in various fields. As technology continues to evolve, and the demand for clean and sustainable energy sources grows, the roles of electrochemical and electrolytic cells in our lives will only become more significant. This underscores the importance of ongoing research and innovation in these fields to meet the energy and environmental challenges of the future.