Chemical reactions are fundamental processes that occur in nature and play a crucial role in various scientific disciplines. Understanding the kinetics of reactions is essential for predicting and controlling the rate at which reactions proceed. Two important concepts in the study of reaction kinetics are the order of reaction and the molecularity of reaction. In this article, we will explore the differences between these two concepts, their definitions, and their implications in chemical kinetics.
Table of Contents
Understanding the Basics of Chemical Reactions
Before delving into the differences between order and molecularity of reaction, let’s first establish a foundation by understanding the basics of chemical reactions. Chemical reactions involve the rearrangement of atoms and molecules to form new substances with different properties. These reactions proceed by breaking existing bonds and forming new ones, resulting in the transformation of reactants into products.
The main difference between order and molecularity of a reaction is that order is a term used to describe the relationship between the concentration of reactants and the rate of the reaction, whereas molecularity refers to the number of molecules that participate in an elementary step of a reaction.
What is Order of Reaction?
Definition
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation. It represents the relationship between the rate of the reaction and the concentration of the reactants. The order can be zero, first, second, or even fractional.
Determining the Order of Reaction
To determine the order of a reaction, one must conduct experiments by varying the concentration of one reactant while keeping the concentrations of other reactants constant. By measuring the rate of reaction under different conditions, it becomes possible to determine the order of the reactant by observing the effect of concentration changes on the reaction rate.
Examples and Illustrations
For instance, consider a hypothetical reaction where reactant A reacts with reactant B to form product C. If the rate of this reaction is directly proportional to the concentration of reactant A, the reaction is said to be first-order with respect to A. Similarly, if the rate is directly proportional to the square of the concentration of reactant B, the reaction is second-order with respect to B.
Let’s consider the reaction between hydrogen gas (H2) and iodine gas (I2) to form hydrogen iodide gas (HI). The balanced chemical equation for this reaction is:
H2(g) + I2(g) → 2HI(g)
The rate of this reaction can be expressed as:
Rate = k[H2]X [I2]Y
Where:
- Rate is the rate of the reaction
- k is the rate constant
- [H2] and [I2] are the concentrations of hydrogen gas and iodine gas, respectively
- x and y are the orders of reaction with respect to hydrogen and iodine, respectively
To determine the order of the reaction, we can perform a series of experiments, varying the initial concentrations of H2 and I2 while keeping the other factors constant. By measuring the rate of the reaction for each experiment, we can determine the values of x and y.
Here’s an example of experimental data:
Experiment 1:
[H2] = 0.1 M
[I2] = 0.2 M
Rate = 0.05 M/s
Experiment 2:
[H2] = 0.2 M
[I2] = 0.2 M
Rate = 0.1 M/s
Experiment 3:
[H2] = 0.1 M
[I2] = 0.4 M
Rate = 0.2 M/s
By comparing the rates of reaction for each experiment, we can determine the order of reaction with respect to H2 and I2. Let’s examine the effect of changing the concentration of H2 while keeping the concentration of I2 constant:
Experiment 1: Rate = 0.05 M/s, Experiment 2: Rate = 0.1 M/s
Since the concentration of H2 doubles (0.1 M to 0.2 M) and the rate of the reaction also doubles, we can conclude that the reaction is first-order with respect to H2 (x = 1).
Now, let’s examine the effect of changing the concentration of I2 while keeping the concentration of H2 constant:
Experiment 1: Rate = 0.05 M/s, Experiment 3: Rate = 0.2 M/s
Here, the concentration of I2 doubles (0.2 M to 0.4 M), but the rate of the reaction quadruples. This indicates that the reaction is second-order with respect to I2 (y = 2).
Therefore, the overall order of reaction for the hydrogen gas and iodine gas reaction is:
Rate = k[H2]1 [I2]2
Keep in mind that this is just an example, and the actual orders of reaction can vary for different reactions.
What is Molecularity of Reaction?
Definition
The molecularity of reaction refers to the number of molecules or atoms that participate in an elementary reaction. An elementary reaction is a single step in a reaction mechanism that describes the detailed molecular-level process of the overall reaction. The molecularity of a reaction can be unimolecular, bimolecular, or termolecular.
Types of Elementary Reactions
In chemical reactions, elementary reactions can involve different numbers of molecules or atoms. Unimolecular reactions involve the decomposition or isomerization of a single molecule. Bimolecular reactions involve the collision and interaction of two molecules, while termolecular reactions involve the simultaneous collision of three molecules.
Determining Molecularity of Reaction
The molecularity of a reaction can be determined by examining the elementary steps involved in the reaction mechanism. An elementary step is a simple, single-step process that describes the collision and interaction of reactant particles. By analyzing the stoichiometry of the reaction and the number of reactant particles involved in each elementary step, one can determine the molecularity of the overall reaction.
Examples
Let’s consider an example to understand the concept of molecularity. The decomposition of hydrogen peroxide (H2O2) can be represented by the following elementary steps:
Step 1: H2O2 → H2O + O (unimolecular)
Step 2: O + H2O2 → H2O + O2 (bimolecular)
In this case, the overall reaction is bimolecular because two molecules of hydrogen peroxide are involved in the second step. The molecularity of the reaction is determined by the slowest step, which in this case is the first step.
Relationship between Molecularity and Order of Reaction
The molecularity of a reaction and the order of reaction are related but distinct concepts. The molecularity describes the stoichiometry of an elementary reaction, whereas the order of reaction describes the dependence of the reaction rate on the concentration of reactants. In some cases, the molecularity of a reaction may be equal to the sum of the orders of reaction with respect to the reactants involved.
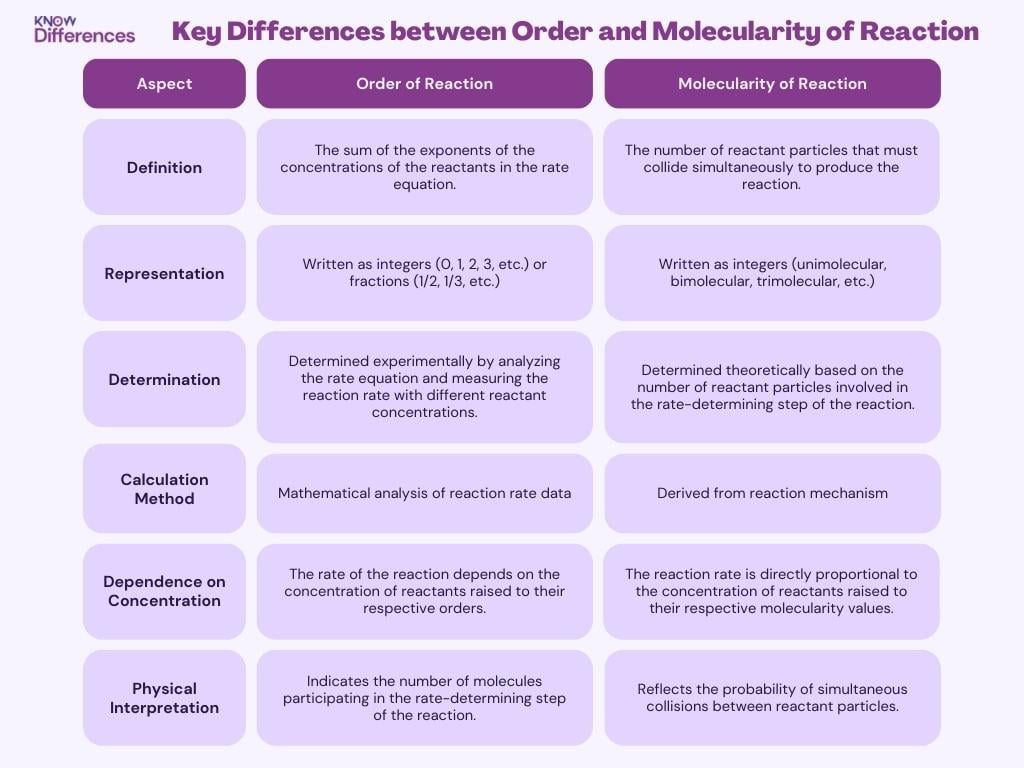
Key Differences between Order and Molecularity of Reaction
Here’s a table highlighting the key differences between order and molecularity of a reaction:
| Order of Reaction | Molecularity of Reaction | |
|---|---|---|
| Definition | The sum of the exponents of the concentrations of the reactants in the rate equation. | The number of reactant particles that must collide simultaneously to produce the reaction. |
| Representation | Written as integers (0, 1, 2, 3, etc.) or fractions (1/2, 1/3, etc.) | Written as integers (unimolecular, bimolecular, trimolecular, etc.) |
| Determination | Determined experimentally by analyzing the rate equation and measuring the reaction rate with different reactant concentrations. | Determined theoretically based on the number of reactant particles involved in the rate-determining step of the reaction. |
| Calculation Method | Mathematical analysis of reaction rate data | Derived from reaction mechanism |
| Dependence on Concentration | The rate of the reaction depends on the concentration of reactants raised to their respective orders. | The reaction rate is directly proportional to the concentration of reactants raised to their respective molecularity values. |
| Physical Interpretation | Indicates the number of molecules participating in the rate-determining step of the reaction. | Reflects the probability of simultaneous collisions between reactant particles. |
| Role in Rate Equation | The order of reaction determines how changes in reactant concentration affect the reaction rate. | The molecularity of reaction provides insights into the mechanism and fundamental steps involved in the reaction. |
| Role in Rate-Determining Step | Helps determine the rate-determining step | Does not directly determine the rate-determining step |
| Relation to Rate Law | Reflects the powers of concentration terms in the rate law equation | Not directly related to the rate law equation |
| Reaction Mechanism | The order of reaction does not provide direct information about the reaction mechanism or the steps involved. | The molecularity of reaction can provide insights into the reaction mechanism, such as whether the reaction occurs in a single step or involves multiple elementary steps. |
| Validity | The order of reaction can change with varying reaction conditions, such as temperature or catalyst presence. | The molecularity of reaction remains constant for a specific reaction under given conditions. |
| Illustrations | Zero order: Rate = k; First order: Rate = k[A]; Second order: Rate = k[A]2; etc. | Unimolecular: Decomposition of ozone (O3 → O2 + O); Bimolecular: Reaction between two molecules (2A + B → products); Trimolecular: Combination of three molecules (A + B + C → products). |
| Examples | Zero order: decomposition reactions First order: radioactive decay, first-order reactions Second order: bimolecular reactions Fractional order: complex reaction | Unimolecular: radioactive decay Bimolecular: elementary step in a two-step reaction Termolecular: rare due to low probability |
Conclusion
In conclusion, the order of reaction and the molecularity of reaction are two key concepts in chemical kinetics that describe different aspects of a chemical reaction. While the order of reaction focuses on the concentration of reactants and its influence on the reaction rate, the molecularity of reaction describes the number of molecules or atoms participating in an elementary reaction. Understanding these differences is vital for comprehending reaction kinetics and predicting the behavior of chemical reactions. By considering both the order and molecularity of a reaction, scientists can gain deeper insights into the mechanisms and rates of chemical transformations.